
2019-nCov Ag&Influenza A/B Ag Rapid Co-Detection Kit
- FOB Price:
- Negotiable | Get Latest Price
- Order Quantity:
- 1 Set / Sets
- Supply Ability:
- 1000 Set / Sets per Month
- Port:
- shanghai
- Payment Terms:
- T/T L/C D/P D/A Credit Card PayPal Cash Escrow Other
- Delivery Detail:
- 5 days
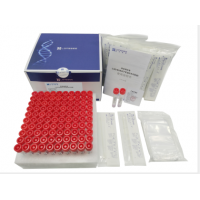
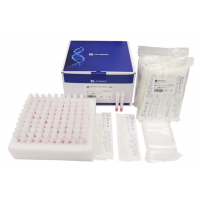
Product Components
| Specification | 1 T/kit | 10 T/kit | ||
|---|---|---|---|---|
| NO. | Name | Major Contents | Qty | Qty |
| 1 | Test Cartridge | PVC, NC membrane, PF cover, Whatsman paper, Microparticle beads conjugated with antibodies. | 1 | 10 |
| 2 |
Saliva Sample Collection Kit (LS-C-M-004) |
Plastic: PP, LDPE Solution: TRIS-EDTA Buffer, DNase and RNase, inhibitor, Preservative, Sodium ion, Surfactant. |
1 | 10 |
Storage Condition & Expiration Date
1. 12 months at 4-35 °C
2. Do not freeze. The test card should be used as soon as possible within 1 hour after the aluminium foil bag is opened.
Instructions for Use
Note: Reading the IFU carefully before using this reagent, and perform testing in strict accordance with the use manual, otherwise reliable results cannot be guaranteed.
1. Before testing
Equilibrate the test cartridge and sample to room temperature before testing. It is recommended to unpack the test card after equilibrating to room temperature and use it as soon as possible within the validity period to prevent the test card from humidity.
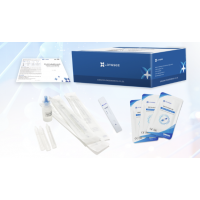
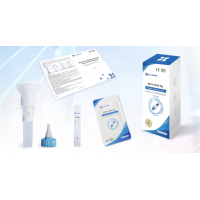
2. Extract
After the subject coughs deeply, spit the expectorated sputum or saliva into the collection funnel gently until the liquid reach 2 mL.Gently turn the saliva collector to make the saliva or sputum flow into the collection tube completely.Tighten the tube cap and attach the label noting the subject information and sampling date after unscrewing the collection funnel carefully. Turn upside down the collection tube slightly for 5 seconds, so that the diluent can mix with the sputum or saliva sample evenly.
3. Loading
Tear off the aluminium foil packaging bag, take out the test card, lay it flat on a horizontal table, drop 3~4 drops (about 100 μL) of the sample vertically into the sample hole, and start timing.
4. Detect
Observe the display result in 15 minutes after adding the sample, and the test result is invalid after 20 minutes.

Interpretation of the Result
1. Invalid: When there is no red line in the quality control area (C), the test is invalid. It is recommended to retest with a new test card, paying particular attention to whether the sample volume is sufficient.
2. Positive: A red line appears in the detection area (T) and the quality control area (C) of the lane COVID-19,Flu A and Flu B respectively.
3. Negative: A red line only appears in the quality control area (C).

-

Stripped Soft Goose Fe
$3.00 -

plastic ball grinding
$30000.00 -

CAT piston pump 281
$4000.00 -
Droichead Zirconia Plu
$10.00 -
E.max crown, Veneer, I
Inquiry -

ReSiC Beams/plates/bur
$16.00 -

RSiC Slabs Boards Tile
$15.00 -

RSiC Batts as Kiln she
$15.00 -

RSiC Tube by recrystal
$10.00 -
RSiC Kiln Furniture (B
$16.00 -

RSiC Burner Nozzle Fla
$18.00 -

RSiC Beam Support Pill
$16.00 -

RSiC plate Slab Board
$15.00 -

NSiC Tube Pipes by Nit
Inquiry -

used excavator hudraul
$16600.00 -

NSiC Thermocouple Prot
Inquiry -

Stalk Riser Tube for L
Inquiry -

NSiC Ceramic Heater Pr
Inquiry -

RSiC NSiC Ceramic Kiln
Inquiry -

used excavator hudraul
$11500.00
- brand:
- Longsee
- Set up shop
- Authorized by Manufacturers & Suppliers online marketplace B2B platform GongWong.com, can provide agency service
- Service Introduction
- Authorized product, Internet cloud promotion service integrating certification promotion and procurement inquiry
- Intelligent website construction
- PC terminal + mobile terminal, create a cost-effective corporate website!